UK Neuralink Trial Participant Hails 'Magical' Brain Chip Experience
In a groundbreaking development, one of the first individuals in the United Kingdom to receive Elon Musk's Neuralink brain chip has described the sensation as 'feels magical', offering renewed optimism for those grappling with severe paralysis. The neurosurgeon spearheading the trial has emphasised that the level of control provided by the chip is 'mindblowing' and poised to be a 'game-changer' in medical technology.
Sebastian Gomez-Pena's Journey and Trial Involvement
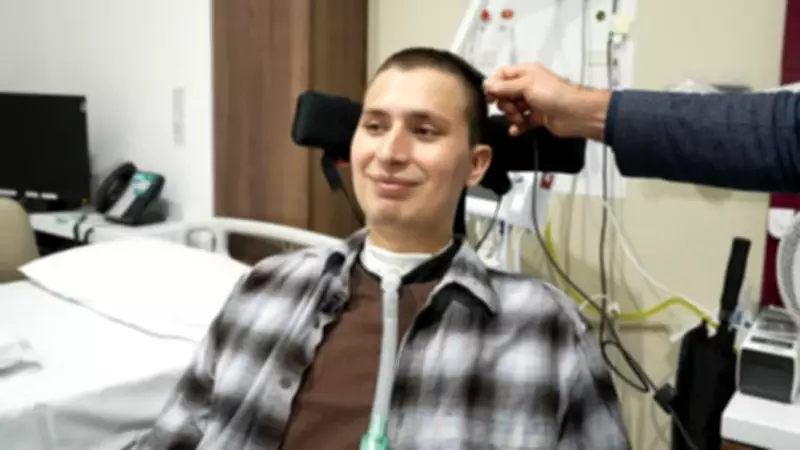
Sebastian Gomez-Pena, a volunteer in the inaugural UK trial of the Neuralink device, shared his personal story, highlighting how the technology has infused him with hope. 'It is a massive change in your life where you can suddenly no longer move any of your limbs,' he recounted, reflecting on the accident that left him paralysed from the neck down shortly after starting medical school. He is among seven participants in the UK trial, which aims to evaluate the safety and reliability of the implant.
The Neuralink chip, connected to 1,024 electrodes implanted in his brain, was surgically placed during a five-hour procedure at University College London Hospital. While British surgeons and Neuralink engineers collaborated, the implantation was executed by Neuralink's R1 robot, designed to insert microscopic electrodes into delicate brain tissue. These electrodes penetrate approximately 4mm into the brain region governing hand movements.
How the Neuralink Technology Functions
Nerve signals are transmitted via threads finer than human hair to the chip, which is embedded in a circular aperture in the skull. Data from the chip is wirelessly relayed to a computer, where artificial intelligence software interprets the signals, translating Seb's intentions into cursor movements on his laptop or phone. 'Everyone in my position tries to move some bit of their body to see if there is any form of recovery, but now when I think about moving my hand it's cool to see that… something actually happens,' he explained. 'You just think it and it does it.'
Observers have noted Seb's ability to navigate a laptop screen with remarkable speed, manipulating text and windows as efficiently as someone using a mouse or touchpad.
Medical Perspectives and Trial Progress
Neurosurgeon Harith Akram, the lead investigator for the UK trial, expressed awe at the control demonstrated by participants. 'It's mindblowing - you can see the level of control that he has,' he remarked, underscoring the promising early results. The technology, developed over nearly two decades, has been implanted in 21 individuals across the United States, Canada, the UK, and the UAE, all suffering from severe paralysis due to spinal injuries, strokes, or neurodegenerative conditions like ALS.
Although results have not yet been published in peer-reviewed journals or submitted to regulators, Mr Akram believes the technology holds transformative potential. 'This technology is going to be a game-changer for patients with severe neurological disability,' he stated, noting its capacity to enhance independence in a technology-dependent world.
Future Applications and Challenges
Neuralink's mission focuses on restoring autonomy and unlocking human potential. Some users have already achieved milestones such as typing on virtual keyboards or controlling robotic arms through thought. Additional trials are exploring speech restoration by targeting brain regions involved in communication, while future initiatives may address blindness by transmitting visual data to the brain.
Elon Musk has envisioned even broader applications, suggesting that users could eventually control Tesla's Optimus robots, effectively inhabiting them for full-body interaction. However, challenges remain, including safely implanting electrodes deeper into the brain and addressing long-term safety and privacy concerns.
Implications and Next Steps
The potential of Neuralink's technology for individuals with paralysis or locked-in syndrome is undeniable, yet it necessitates further large-scale trials to ensure safety and reliability before widespread licensing. As the trial advances, volunteers like Sebastian Gomez-Pena play a crucial role in realising this innovative medical breakthrough.





