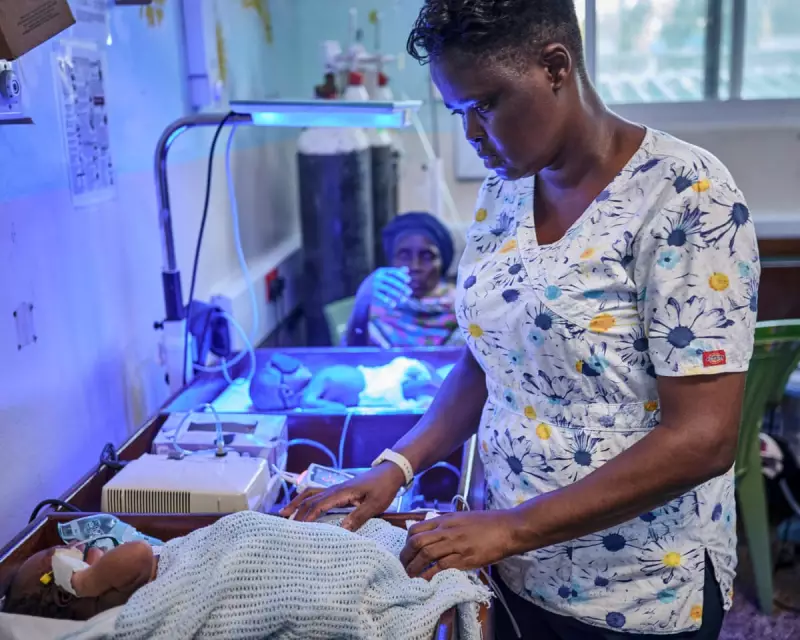
In a quiet corner of Kenya, far from the tourist trails of Kilifi Creek, a medical revolution is quietly unfolding that could save countless newborn lives worldwide. The NeoSep1 clinical trial represents a pioneering effort to combat one of the most devastating threats to infant health: neonatal sepsis.
The Silent Killer Claiming 800,000 Newborn Lives Annually
Sepsis, a life-threatening infection, claims approximately 800,000 newborn lives each year globally. The situation is particularly dire in Africa, where this condition accounts for 28% of all neonatal deaths. Newborns are especially vulnerable because their underdeveloped immune systems struggle to fight off pathogens effectively.
The crisis has been exacerbated by growing antibiotic resistance. According to recent estimates, up to 214,000 neonatal deaths worldwide are now caused by antimicrobial-resistant infections. Many infants die because the standard antibiotics recommended for treating sepsis have become ineffective as bacteria evolve and develop resistance.
A Global Response to a Growing Crisis
The NeoSep1 trial, led by the Global Antibiotic Research and Development Partnership (GARDP), represents a coordinated international effort to address this emergency. The study is running across eight countries: South Africa, Kenya, Ghana, India, Bangladesh, Pakistan, Malaysia and Vietnam. By 2029, the trial aims to enrol 3,000 babies, including 600 newborns aged up to 28 days at three Kenyan clinics in Mombasa, Nairobi and Kilifi.
Christina Obiero, principal investigator of the trial at Kilifi County Hospital, explains the significance of their work: "We were excited to find that the dosages we were using are safe and effective. In the second part of the trial, these treatments will be further used to treat sepsis in newborns."
Repurposing Old Drugs for New Solutions
One of the most innovative aspects of the NeoSep1 trial involves repurposing older antibiotics that have never been used in Africa to treat sepsis. The research focuses on two specific drugs: fosfomycin and flomoxef.
Fosfomycin, developed in the 1970s, is widely used in Europe for urinary tract infections and increasingly as part of combination therapies for difficult-to-treat infections. Flomoxef, from the 1980s, has been used almost exclusively in east Asia. Now that these drugs are generic, they have become much more affordable for widespread use.
Sally Ellis, project leader of GARDP's children's antibiotics programme, emphasises the strategic approach: "We aim to improve the dosing information of each antibiotic combination, identify the type of organisms that are causing the infection, and find potential or new treatment options."
The research team has narrowed down the treatment options to eight antibiotic combinations, including first- and second-line treatments alongside newer options like fosfomycin paired with flomoxef. Alexander Makazi, study coordinator at the Kenya Medical Research Institute, describes their methodology: "We exposed the bacteria to a panel of different antibiotics... If it was unable to spread, we knew that it was being killed by that specific drug."
The Race Against Time in Sepsis Treatment
Speed is critical when treating neonatal sepsis. The infection can develop suddenly, and a newborn's condition can deteriorate within seconds, making rapid response and prompt initiation of antibiotics essential. However, identifying the specific bacteria affecting a baby can take approximately five days from when the blood sample is taken until laboratory results are available.
Robert Mwakesi, manager of the Kemri-Wellcome Trust Research Programme laboratory adjacent to Kilifi County Hospital's high-dependency unit, highlights this challenge. The medical team cares for babies under the watchful eyes of their families while awaiting crucial test results.
Obiero stresses the importance of their work: "If we know exactly which bacteria are causing the infection and which treatment works best, we can act quickly and save more lives."
Transforming Global Treatment Guidelines
In Africa, NeoSep1 forms part of a five-year project called Snip-Africa, bringing together health professionals from multiple sectors working toward the same goal: ranking the eight antibiotic combinations to determine which are most effective and safest for each newborn presenting with sepsis.
The ultimate objective extends beyond identifying the best treatment options. Obiero explains: "Once we have the results of the ranking we aim to inform the World Health Organization and national governments to revise the existing guidelines. Beyond identifying the best option for each infection, the goal is also to minimise exposing babies to unnecessary antibiotics, because that is what ultimately drives resistance."
Makazi recalls a powerful moment that underscores the human impact of their work: "The look on the mother's face when she saw the improvement in her sick baby – now strong enough to latch on to her breast – was incredibly moving. Moments like these remind us that our work is truly making a difference."
As the NeoSep1 trial continues across multiple continents, it represents not just a scientific endeavour but a beacon of hope for families worldwide facing the terrifying prospect of neonatal sepsis. The research promises to transform treatment protocols and save countless newborn lives through the innovative repurposing of existing medical resources.





