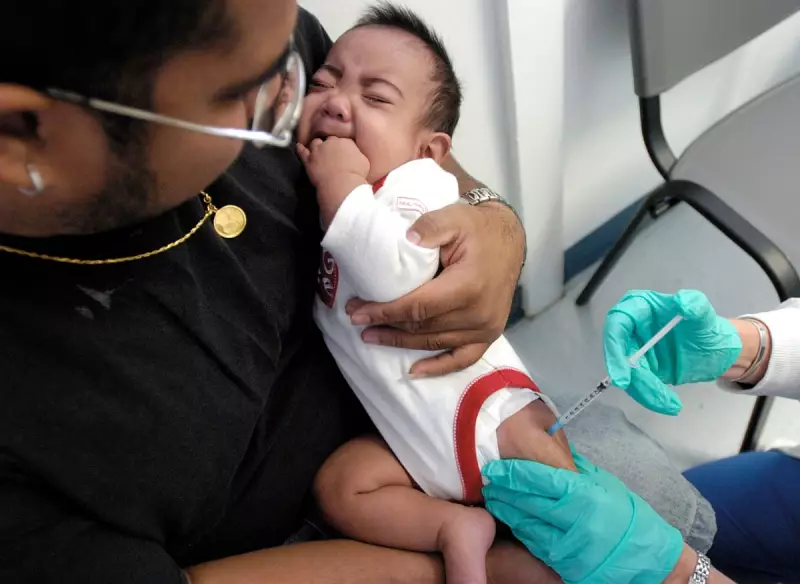
Public health experts in the United States are raising the alarm as the Trump administration moves to restrict access to a highly effective vaccine against Respiratory Syncytial Virus (RSV) for newborns, a decision that flies in the face of compelling new evidence showing the jab's power to prevent severe illness.
New Restrictions Clash with Mounting Evidence
Last week, US Health Secretary Robert F Kennedy Jr., a prominent vaccine critic, announced new limitations covering one-third of all routine childhood immunisations. Among them, the RSV shot for infants is now recommended only for babies deemed high-risk, rather than all newborns. This policy shift comes as four new studies published in the Journal of the American Medical Association (JAMA) confirm earlier observations of a significant decline in hospitalisations thanks to the vaccine.
Infections from RSV, a virus particularly dangerous for young children, are beginning to increase in the current US respiratory season. Prior to the approval of these shots in 2023, between 2% and 3% of all babies in the US were hospitalised with the virus. "It's the most common reason for children under five to be hospitalized," said Kevin Ault, an obstetrician-gynaecologist and former member of the CDC's RSV working group.
Why a Universal Approach is Deemed Critical
The decision to limit the vaccine has baffed many medical professionals, given the virus's profile. Crucially, 81% of babies hospitalised with RSV have no underlying health conditions. Restricting the shot only to children with existing issues will therefore "miss a large majority of the potential cases," warns Dr Ault. "That's very concerning, and that's why a universal recommendation was made," he added.
The data supporting widespread use is robust. One study found the RSV vaccination during pregnancy is 70% effective at preventing infant hospitalisation, while the monoclonal antibody shot given directly to newborns was 81% effective. Another study noted the infant shot also appears to prevent hospitalisation from all lower-respiratory infections, likely because RSV can cause lingering effects like bacterial infections.
Professor Richard Rupp of the University of Texas Medical Branch, who was involved in the vaccine trials, described the tangible impact: "It's easy to see in real life. We can really tell that hospitalisations are down. It's made a big difference." He called RSV "a horrible disease" that can rapidly turn a mild cold into a life-threatening fight for breath.
Fragmented System and Future Confusion
The new policy is expected to create significant practical problems and deepen health inequalities. Dr Ault criticised the move as "made by political appointees without a scientific basis." He and others fear the restrictions will erode access, even for babies who still qualify.
"If you start having [more] fragmentation of the system, you're going to see hospitals and offices not stocking it," Ault explained. "So even if it's covered on paper, it might not be available." This could lead to dangerous confusion during the RSV season, with paediatricians and hospitals unsure who is responsible for administering the jab.
Furthermore, the definition of "high-risk" remains unclear. Given the high hospitalisation rate among otherwise healthy infants, both Rupp and Ault argue that simply being a baby in the first few months of life constitutes high risk. "All babies in the first few months of life are at high risk for RSV," Ault stated.
The decision also places coverage under schemes like the federal Vaccines for Children programme in doubt. "Those are the people who really will not be able to afford to have this for their children," Professor Rupp said, highlighting the potential for the move to disproportionately hurt the most vulnerable families.





